Fill This Form To Receive Instant Help
Fill This Form To Receive Instant Help
Homework answers / question archive / Consider the following Gibbs energies at 25 °C
Consider the following Gibbs energies at 25 °C.
Substance Ag (aq) Cl–(aq) AgCl(s) Br–(aq) AgBr(s)
(a) Calculate ?G°rxn for the dissolution of AgCl(s).
(b) Calculate the solubility-product constant of AgCl.
(c) Calculate ?G°rxn for the dissolution of AgBr(s).
(d) Calculate the solubility-product constant of AgBr.
| Substance | 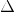 Gf Gf |
| Ag+ | 77.1 |
| Cl- | -131.2 |
| AgCl | -109.8 |
| Br- | -104 |
| AgBr | -96.9 |
Already member? Sign In